Matter
Everything in the universe is made of matter, which occupies space and has mass — meaning it has both mass and volume. Scientists classify matter into two types based on physical properties and chemical nature.
Characteristics of Particles of Matter:
Particles have space between them and are in continuous motion. When temperature rises, their kinetic energy increases, making particles move faster.
- The intermixing of particles of two different types of matter on their own is called diffusion. Heating speeds up diffusion.
States of Matter & Diffusion
Matter exists in three states – solid, liquid, and gas Liquids diffuse faster than liquids and solids because their particles have greater freedom of movement and more space between them.
- Diffusion rate: Gases > Liquids > Solids
Compressibility of Gases
Gases are highly compressible compared to solids and liquids. This property allows Large volumes of gas to be stored in small containers. Common examples include LPG cylinders, oxygen cylinders in hospitals, and CNG used as vehicle fuel.
Q. What is matter? (A) Anything that occupies space & has Volume (B) Anything that occupies space & has mass (C) Only gases (D) Only solids
Q. Diffusion is the process of – (A) Cooling of particles (B) Intermixing of particles (C) Freezing of matter (D) Melting of solids
Q. 1 millilitre (ml) is equal to : (A) 1 liter (B) 1 cubic centimetre (C) 1 cubic metre (D) 1000 litres
Q. Which state of matter has the highest rate of diffusion? (A) Solid (B) Liquid (C) Gas (D) Plasma
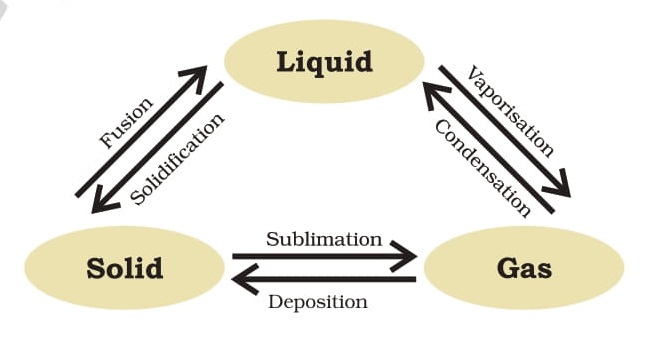
The minimum temperature at which a solid melts to become a liquid at the atmospheric pressure is called its melting point. The melting point of ice is 273.15 K. The process of melting, that is, change of solid state into liquid state is also known as fusion.
You must have observed, during the experiment of melting, that the temperature of the system does not change after the melting point is reached, till all the ice melts. This happens even though we continue to heat the beaker, that is, we continue to supply heat. This heat gets used up in changing the state by overcoming the forces of attraction between the particles. As this heat energy is absorbed by ice without showing any rise in temperature, it is considered that it gets hidden into the contents of the beaker and is known as the latent heat. The word latent means hidden.
The amount of heat energy that is required to change 1 kg of a solid into liquid at atmospheric pressure at its melting point is known as the latent heat of fusion. So, particles in water at 0°C (273 K) have more energy as compared to particles in ice at the same temperature.
The temperature at which a liquid starts boiling at the atmospheric pressure is known as its boiling point. Boiling is a bulk phenomenon. Particles from the bulk of the liquid gain enough energy to change into the vapour state. For water this temperature is 373 K (100°C-273+100=373 К).
Particles in steam, that is, water vapour at 373 K (100°C) have more energy than water at the same temperature. This is because particles in steam have absorbed extra energy in the form of latent heat of vaporisation.
“Note: Kelvin is the SI unit of temperature, 0°C -273.15 K. For convenience, we take 0°C = 273 K after rounding off the decimal. To change a temperature on the Kelvin scale to the Celsius scale you have to subtract 273 from the given temperature, and to convert a temperature on the Celsius scale to the Kelvin scale you have to add 273 to the given temperature.
A change of state directly from solid to gas without changing into liquid state is called sublimation and the direct change of gas to solid without changing into liquid is called deposition.
Applying pressure and reducing temperature can liquefy gases. Have you heard of solid carbon dioxide (CO)? It is stored under high pressure. Solid CO, gets converted directly into gaseous state on decrease of pressure to 1 atmosphere without coming into liquid state. This is the reason that solid carbon dioxide is also known as dry ice.
Thus, we can say that pressure and temperature determine the state of a substance, whether it will be solid, liquid or gas.
Interconversion of the three states of matter
atmosphere (atm) is a unit of measuring pressure exerted by a gas. The unit of pressure is Pascal (Pa): 1 atmosphere 1.01 10 Pa. The pressure of air in atmosphere is called atmospheric pressure. The atmospheric pressure at sea level is 1 atmosphere, and is taken as the normal atmospheric pressure.
We know that particles of matter are always moving and are never at rest. At a given temperature in any gas, liquid or solid, there are particles with different amounts of kinetic energy. In the case of liquids, a small fraction of particles at the surface, having higher kinetic energy, is able to break away from the forces of attraction of other particles and gets converted into vapour. This phenomenon of change of liquid into vapours at any temperature below its boiling point is called evaporation.
1.5.1 FACTORS AFFECTING EVAPORATION
Let us understand this with an activity.
Activity
1.14
Take 5 mL of water in a test tube and keep it near a window or under a fan.
Take 5 mL of water in an open china dish and keep it near a window or under a fan.
Take 5 ml of water in an open china dish and keep it inside a cupboard or on a shelf in your class.
MATTER IN OUR SURROUNDINGS
Record the room temperature.
Record the time or days taken for the evaporation process in the above cases.
Repeat the above three steps of activity on a rainy day and record your observations.
What do you infer about the effect of temperature, surface area and wind velocity (speed) on evaporation?
You must have observed that the rate of evaporation increases with-
an increase of surface area:
We know that evaporation is a surface phenomenon. If the surface area is increased, the rate of evaporation increases. For example, while putting clothes for drying up we spread them out.
an increase of temperature:
With the increase of temperature, more number of particles get enough kinetic energy to go into the vapour state.
a decrease in humidity:
Humidity is the amount of water vapour present in air. The air around us cannot hold more than a definite amount of water vapour at a given temperature. If the amount of water in air is already high, the rate of evaporation decreases. an increase in wind speed:
It is a common observation that clothes dry faster on a windy day. With the increase in wind speed, the particles of water vapour move away with the wind, decreasing the amount of water vapour in the surrounding.
1.5.2 HOW DOES EVAPORATION CAUSE COOLING?
In an open vessel, the liquid keeps on evaporating. The particles of liquid absorb energy from the surrounding to regain the energy lost during evaporation. This absorption of energy from the surroundings make the surroundings cold.
What happens when you pour some acetone (nail polish remover) on your palm? The particles gain energy from your palm or surroundings and evaporate causing the palm to feel cool.
After a hot sunny day. people sprinkle water on the roof or open ground because the large latent heat of vaporisation of water helps to cool the hot surface.
Can you cite some more examples from daily life where we can feel the effect of cooling due to evaporation?
Why should we wear cotton clothes in summer?
During summer, we perspire more because of the mechanism of our body which keeps us cool. We know that during evaporation, the particles at the surface of the liquid gain energy from the surroundings or body surface and change into vapour. The heat energy equal to the latent heat of vaporisation is absorbed from the body leaving the body cool. Cotton, being a good absorber of water helps in absorbing the sweat and exposing it to the atmosphere for easy evaporation.
Why do we see water droplets on the outer surface of a glass containing ice-cold water?
Let us take some ice-cold water in a tumbler. Soon we will see water droplets on the outer surface of the tumbler. The water vapour present in air, on coming in contact with the cold glass of water, loses energy and gets converted to liquid state, which we see as water droplets.
uestions
1. Why does a desert cooler cool better on a hot dry day?
2. How does the water kept in an earthen pot (matka) become cool during summer?
3. Why does our palm feel cold when we put some acetone or petrol or perfume on it? Why are we able to sip hot tea or milk faster from a saucer rather than a cup?
NCERT
5. What type of clothes should we wear in summer?
repu
What you have learnt
be
to
Matter is made up of small particles.
The matter around us exists in three states-solid. liquid and gas.
not
10
The forces of attraction between the particles are maximum in solids, intermediate in liquids and minimum in gases.
The spaces in between the constituent particles and kinetic energy of the particles are minimum in the case of solids, intermediate in liquids and maximum in gases.
SCIENCE
Reprint 2025-26
The arrangement of particles is most ordered in the case of solids, in the case of liquids layers of particles can slip and slide over each other while for gases, there is no order, particles just move about randomly.
The states of matter are inter-convertible. The state of matter can be changed by changing temperature or pressure.
Sublimation is the change of solid state directly to gaseous state without going through liquid state.
Deposition is the change of gaseous state directly to solid state without going through liquid state.
Boiling is a bulk phenomenon. Particles from the bulk (whole) of the liquid change into vapour state.
Evaporation is a surface phenomenon. Particles from the surface gain enough energy to overcome the forces of attraction present in the liquid and change into the vapour state.
The rate of evaporation depends upon the surface area exposed to the atmosphere, the temperature, the humidity and the wind speed.
Evaporation causes cooling.
b
Latent heat of vaporisation is the heat energy required to change 1 kg of a liquid to gas at atmospheric pressure at its boiling point.
Latent heat of fusion is the amount of heat energy required to change 1 kg of solid into liquid at its melting point.
Some measurable quantities and their units to remember:

Exercises
1. Convert the following temperatures to the celsius scale.
(a) 293 K
(b) 470 K
2. Convert the following temperatures to the kelvin scale.
(a) 25° C
(b) 373° C
3. Give reason for the following observations.
(a) Naphthalene balls disappear with time without leaving any solid.
(b) We can get the smell of perfume sitting several metres away.
4. Arrange the following substances in increasing order of forces of attraction between the particles- water, sugar, oxygen.
5. What is the physical state of water at-
(a) 25° C
(b) 0° C
(c) 100° C?
6. Give two reasons to justify-
(a) water at room temperature is a liquid.
(b) an iron almirah is a solid at room temperature.
7. Why is ice at 273 K more effective in cooling in cooling than water at the same temperature?
8. What produces more severe burns, boiling water or steam?
following diagram showing 9. Name A,B,C,D,E and F in the follow
change in its state N
Increase heat and decrease pressure
SOLID
LIQUID
F
C
GAS
not to
12
Decrease heat and Increase pressure
E
SCIENCE
Reprint 2025-26
